Tucked away on the fifth floor of Shepherd Laboratories at the University of Minnesota is the Medical Devices Center âÄî a playground for anyone in the fields of biomedical or chemical engineering.
Begun from a fund that supports special initiatives on campus, the Medical Devices Center was part of a strategic positioning process started by University administration.
âÄú[University administration] was persuaded that the University had been doing a really good job in clinical research and basic science research,âÄù Arthur Erdman, director of the Center, said. âÄúThe missing piece was the translation of biomedical research into projects.âÄù
Erdman, who had been pushing the University for a similar medical device center, was awarded a five-year, $5 million investment for the Center in 2006.
âÄúWhat I try to do here, and it wasnâÄôt necessarily received by all my colleagues, is a good idea,âÄù Erdman said.
Erdman designed the Center to include a machine and electrical workshop. Prior to 2006, similar workshops had been housed on campus, but in separate buildings.
By combining everything in one place, researchers could design and test their devices without ever leaving the fifth floor, Erdman said. In the past five years, the center has funded about 30 projects, including 12 in 2009.
The Medical Devices Center has increased to include 4,000 square feet of work space, taking over the entire fifth floor of Shepherd Laboratories.
âÄúItâÄôs an unusual resource for the University to have a group of people like this that have the engineering background and the access to equipment to allow rapid fabrication of either a prototype or a finished device,âÄù Steven Rothman, director of Clinical Neuroscience in Pediatrics, said.
Rothman brought to the Medical Devices Center a prototype of an implantable cooling grid that could prevent seizures.
âÄúThey had tried this in animal trials and they were looking to refine the prototype and make it more appealing to the [Food and Drug Administration] to use in human trials,âÄù Lucas Harder, a graduate student and lab supervisor, said.
Harder and the team at the Medical Devices Center helped Rothman customize the cooling grid so it could be approved by the FDA. RothmanâÄôs device was originally designed using a number of parts that were not FDA approved. Through the Medical Devices Center, Rothman was able to assemble the device using approved equipment.
âÄúWe provide space, equipment and expertise as much as we can,âÄù Harder said. âÄúItâÄôs a lot easier to get things through the FDA that [are] already FDA approved than making something from scratch.âÄù
Without the help of the Medical Devices Center, it would have been necessary for Rothman to work with a private vendor.
âÄúHere we have all these [FDA-approved devices] and weâÄôve seen all these things before so we whipped a prototype together and tried it,âÄù Harder said.
The cooling grid device is currently in its third version and will soon undergo the FDA approval process. Rothman believes human trials will take place within the year.
âÄúIf individual investigators had to assemble all the tools that they would need to do all this kind of work, it would take months or even years,âÄù Rothman said. âÄúThe fact that all these resources are readily available greatly speeds the time of development of some new device.âÄù
The initial five years of funding for the center will run out in July, but the center has saved enough money to continue for at least another year.
The Medical Devices Center has also attracted a lot of industry attention and funding, according to Erdman.
Boston Scientific and St. Jude Medical, both of which manufacture medical devices, are among a list of private contributors to the center, donating money and equipment. Some of the devices found at the Medical Devices Center are one-of-a-kind on campus.
One of the âÄúgemsâÄù at the Medical Devices Center is a 3D television that fiber-optically streams data from the operating room where it can be viewed in 3D in one of the centerâÄôs meeting rooms.
âÄúAs engineers when youâÄôre working in a biomedical space, a lot of times youâÄôre looking over a doctorâÄôs shoulders trying to get a better look,âÄù Harder said. âÄúItâÄôs really hard to get good data because youâÄôre always in the way.âÄù
The Medical Devices CenterâÄôs goal is to affect health care and improve devices to make them more functional at a lower cost, Erdman said.
Harder and Erdman have given more than 200 tours in the past two years to guests ranging from private sector representatives to University alumni.
âÄúThis is a very unique place in the country,âÄù Erdman said. âÄúI think if there was something like this elsewhere, we would have heard about it already.âÄù
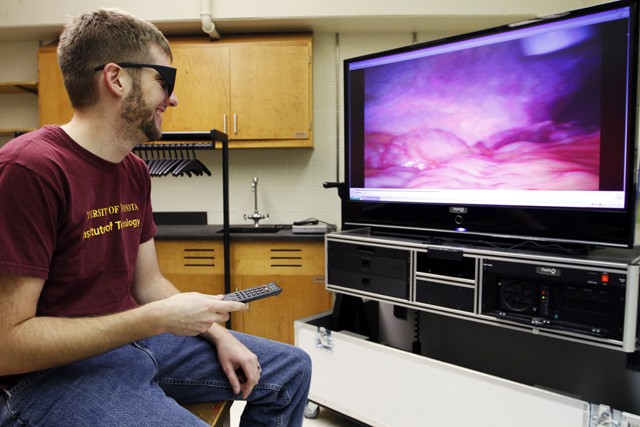
Image by Joe Michaud-Scorza
Graduate student and Medical Devices Center lab supervisor Lucas Harder watches a recording on a 3D surgical observation television Monday in Shepherd Laboratories. The TV receives live 3D video via fiber optics from surgeries being preformed across the university.
From research to the real world
The Medical Devices Center has given life to more than 30 projects in five years.
by Frank
Published October 5, 2010
0

