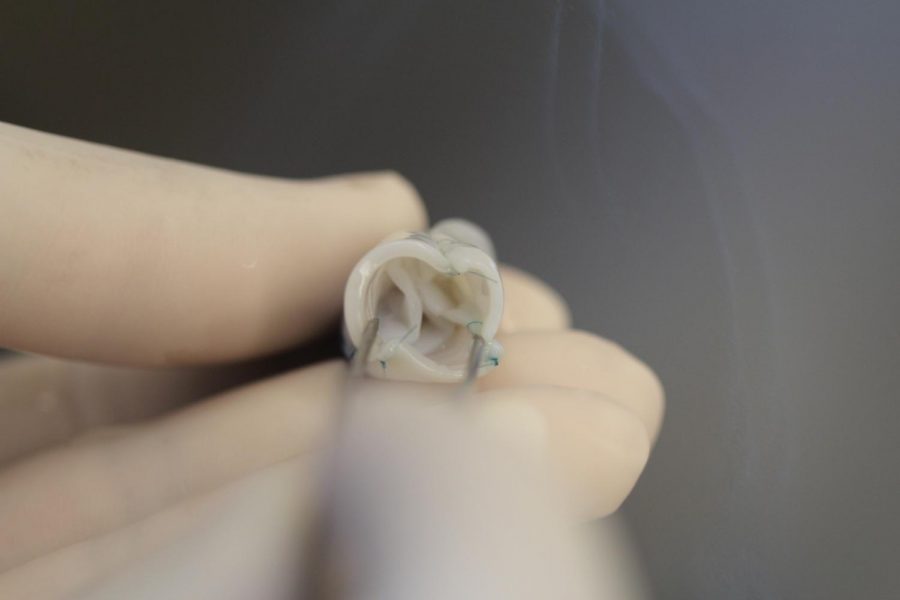
University of Minnesota researchers are conducting animal trials for a newly developed synthetic heart valve, which can grow with its recipient.
These valves can be used for those with congenital heart defects (CHDs), the most common type of birth defect, affecting about 40,000 babies in the United States each year, according to the Centers for Disease Control and Prevention. These heart defects are present at birth and can affect the function of a baby’s heart.
Currently, the only options for children with CHDs are valves that can defect or require numerous open-heart surgeries as the child outgrows the valve. Pediatric patients are lucky to get five years of use out of current devices, University biomedical engineering researcher Zeeshan Syedain said.
“The problem right now is that none of the materials grow,” said Dr. Robert Tranquillo, a professor in the Department of Biomedical Engineering.
Researchers from the University’s Medical School and College of Science and Engineering developed this new valve and recently concluded a yearlong study on its efficacy. The study was published earlier this month in Science Translational Medicine, a medical journal by the American Association for the Advancement of Science.
The valve has shown its ability to grow with lamb recipients, giving the implanted tissue the same living capabilities as real tissue, Syedain said.
“We’re going to use this to develop valves that are more durable, that can last longer and that can be part of your own body rather than a foreign material, which is what they are now,” Richard Bianco, a professor of surgery at the Medical School, said.

Syedain said the researchers’ hope is to replace tissue that has stopped functioning or never properly functioned with their synthetic tissue that will work for the rest of the patient’s life.
Since implanting their first sheep in 2017, University researchers have made substantial improvements in both material and design, Tranquillo said.
This valve-making technology has been patented and licensed to Vascudyne, a University start-up company for which Tranquillo is a consultant.
The researchers chose lambs for testing because of their size and quick transition to adulthood. These factors allowed researchers to use clinical-sized devices and measure synthetic valve growth over a short time.
“We need to mimic the clinical intent as close as possible,” Bianco said.
To create the synthetic valve, researchers used a single donor lamb’s cells. They removed the donor cells from the tissue and created a material that any recipient sheep can use. When the new material was implanted in the recipient sheep, it did not induce a negative immune response, Syedain said.
“This has been the holy grail,” Bianco said.
After they implanted the new valve, the recipient lamb’s cells started to populate the valve, allowing it to grow with them.
“You have a material that is essentially a cell product but is something that is cellular and can be stored and implanted into any patient,” Tranquillo said.
This material can be preprepared and stored for at least six months. The technology also has the potential to be used for vascular grafts for people with dialysis, kidney disease or peripheral artery disease or for those in need of a bypass graft, Syedain said.
In this most recent study, three of the seven sheep’s valve implants were deemed successful after a year, meaning the valves did not leak or experience blockages.
The researchers are conducting another yearlong sheep study, which will resemble a clinical trial as closely as possible. Bianco said the team hopes to get the Food and Drug Administration’s approval to begin human trials.
“I want the device that would go into a human, and I want to test that device. So a lot more regulations but essentially the same study with more animals,” Bianco said.
With the FDA’s approval, proper funding and a team including pediatric surgeons to implement the valve, researchers could start human trials within a year or two, Tranquillo said. The researchers could conduct a clinical trial observing the synthetic valve’s use in three to four years.
“But there’s a lot of milestones to be achieved before that,” Tranquillo said.